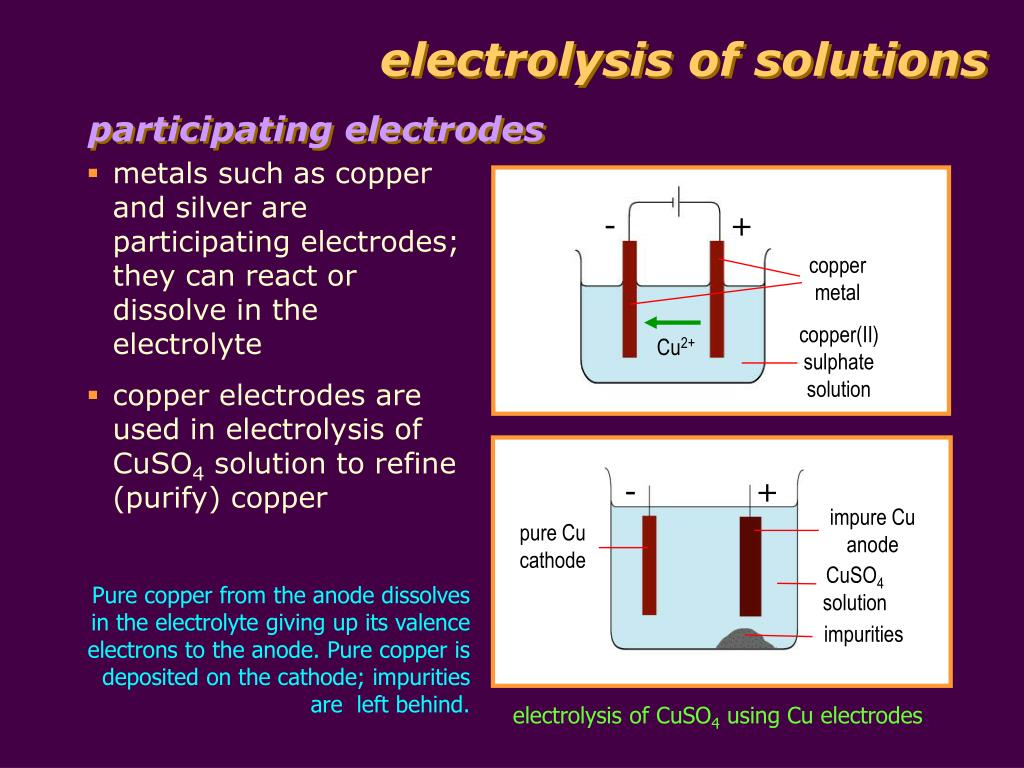
Inert Electrode Chemistry Definition . An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. Graphite(carbon), platinum, gold, and rhodium. examples of electrodes. an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. This is an electrode that is made of a substance that does not undergo oxidation or reduction. Some commonly used inert electrodes: the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes.
from www.slideserve.com
An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Some commonly used inert electrodes: an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. examples of electrodes. This is an electrode that is made of a substance that does not undergo oxidation or reduction. Graphite(carbon), platinum, gold, and rhodium.
PPT electrolysis of solutions PowerPoint Presentation, free download
Inert Electrode Chemistry Definition This is an electrode that is made of a substance that does not undergo oxidation or reduction. Graphite(carbon), platinum, gold, and rhodium. An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. examples of electrodes. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Some commonly used inert electrodes: an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. This is an electrode that is made of a substance that does not undergo oxidation or reduction.
From www.slideserve.com
PPT Lets Set The Stage PowerPoint Presentation, free download ID Inert Electrode Chemistry Definition examples of electrodes. an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Graphite(carbon), platinum, gold, and rhodium. Some commonly. Inert Electrode Chemistry Definition.
From studycopesettic.z21.web.core.windows.net
What Is An Inert Electrode Inert Electrode Chemistry Definition An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. examples of electrodes. Some commonly used inert electrodes: Graphite(carbon), platinum, gold, and rhodium. This is an electrode that is made of a substance that does not undergo oxidation or reduction. An inert electrode is a conductive material that does not participate. Inert Electrode Chemistry Definition.
From icsechemistry16.blogspot.com
Electrolysis of Copper sulphate using inert electrodes Inert Electrode Chemistry Definition An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Graphite(carbon), platinum, gold, and rhodium. Some commonly used inert electrodes: An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. the main difference between active and inert electrodes is that active electrodes participate. Inert Electrode Chemistry Definition.
From www.youtube.com
Chemistry Electrolysis (Inert & Active Electrodes) YouTube Inert Electrode Chemistry Definition This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. An inert electrode is a conductor that does not participate in the. Inert Electrode Chemistry Definition.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation ID4966899 Inert Electrode Chemistry Definition examples of electrodes. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. An inert electrode is a conductive material. Inert Electrode Chemistry Definition.
From www.differencebetween.com
Difference Between Active and Inert Electrodes Active vs Inert Electrodes Inert Electrode Chemistry Definition An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. This is an electrode that is made of a substance that does not undergo oxidation or reduction. Graphite(carbon), platinum, gold, and rhodium. examples of electrodes. an inert electrode is a conductor that does not participate in any electrochemical reactions during. Inert Electrode Chemistry Definition.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate Inert Electrode Chemistry Definition This is an electrode that is made of a substance that does not undergo oxidation or reduction. Some commonly used inert electrodes: An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in. Inert Electrode Chemistry Definition.
From www.youtube.com
Inert electrodes Trew’s notes YouTube Inert Electrode Chemistry Definition an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Graphite(carbon), platinum, gold, and rhodium. An inert electrode is a conductor. Inert Electrode Chemistry Definition.
From brainly.in
There are two types of electrodes and they are Active electrode Inert Inert Electrode Chemistry Definition Graphite(carbon), platinum, gold, and rhodium. This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. An inert electrode is a conductor that. Inert Electrode Chemistry Definition.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Inert Electrode Chemistry Definition An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. examples of electrodes. This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main. Inert Electrode Chemistry Definition.
From www.slideserve.com
PPT Chemistry Comes Alive PowerPoint Presentation, free download ID Inert Electrode Chemistry Definition This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. an inert electrode is a conductor that does not participate in. Inert Electrode Chemistry Definition.
From dxontbmym.blob.core.windows.net
Electrode Meaning Chemistry at Christy Rogers blog Inert Electrode Chemistry Definition examples of electrodes. an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. Some commonly used inert electrodes: This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference between active and inert electrodes is that active electrodes participate in the. Inert Electrode Chemistry Definition.
From www.slideshare.net
Electrolysis part 3 aqueous solution Inert Electrode Chemistry Definition examples of electrodes. Some commonly used inert electrodes: an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. the main difference between active and inert electrodes is that active electrodes participate in. Inert Electrode Chemistry Definition.
From quiztetrasemic.z21.web.core.windows.net
Examples Of Inert Electrodes Inert Electrode Chemistry Definition an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Graphite(carbon), platinum, gold, and rhodium. examples of electrodes. This is an electrode that is made of a substance that does not undergo oxidation. Inert Electrode Chemistry Definition.
From chem.libretexts.org
6.2 Standard Electrode Potentials Chemistry LibreTexts Inert Electrode Chemistry Definition Graphite(carbon), platinum, gold, and rhodium. Some commonly used inert electrodes: the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. . Inert Electrode Chemistry Definition.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Inert Electrode Chemistry Definition an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. Some commonly used inert electrodes: This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in. Inert Electrode Chemistry Definition.
From www.youtube.com
ICSE Std10 Chemistry Role of Active Electrode & Inert Electrode CuSO4 Inert Electrode Chemistry Definition the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. An inert electrode is a conductive material that does not participate in the chemical reaction of an electrochemical. Some commonly used inert electrodes: examples of electrodes. an. Inert Electrode Chemistry Definition.
From www.slideserve.com
PPT Group 13 Compounds PowerPoint Presentation, free download ID Inert Electrode Chemistry Definition An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. examples of electrodes. an inert electrode is a conductor that does not participate in any electrochemical reactions during the electrodeposition. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in. Inert Electrode Chemistry Definition.